-
5.3.2:
For patients without an increased bleeding risk or impaired coagulation and not already receiving effective systemic anticoagulation, we suggest the following:
- 5.3.2.1: For anticoagulation in intermittent RRT, we recommend using either unfractionated or low-molecular-weight heparin, rather than other anticoagulants. (1C)
- 5.3.2.2: For anticoagulation in CRRT, we suggest using regional citrate anticoagulation rather than heparin in patients who do not have contraindications for citrate. (2B)
- 5.3.2.3: For anticoagulation during CRRT in patients who have contraindications for citrate, we suggest using either unfractionated or low-molecular-weight heparin, rather than other anticoagulants. (2C)
Rationale
Worldwide, unfractionated heparin is still the most widely used anticoagulant. Many European centers, however, have switched from unfractionated to low-molecular-weight heparin for routine anticoagulation during IHD.579 Advantages and disadvantages of each type of heparin are summarized in Table 19.
A recent meta-analysis of 11 RCTs comparing unfractionated to low-molecular-weight heparin in chronic IHD concluded that both are equally safe in terms of bleeding complications (RR 0.96; CI 0.27–3.43) and as effective in preventing extracorporeal thrombosis (RR 1.15; CI 0.7–1.91).586 Mainly because of the convenience of using a single bolus injection at the start of IHD, the reduced risk of heparin-induced thrombocytopenia (HIT), and of long-term side-effects such as abnormal serum lipids, osteoporosis, and hypoaldosteronism, the European practice guideline for prevention of dialyzer clotting suggests using low-molecular-weight rather than unfractionated heparin in chronic dialysis patients.587 Many European centers have extrapolated
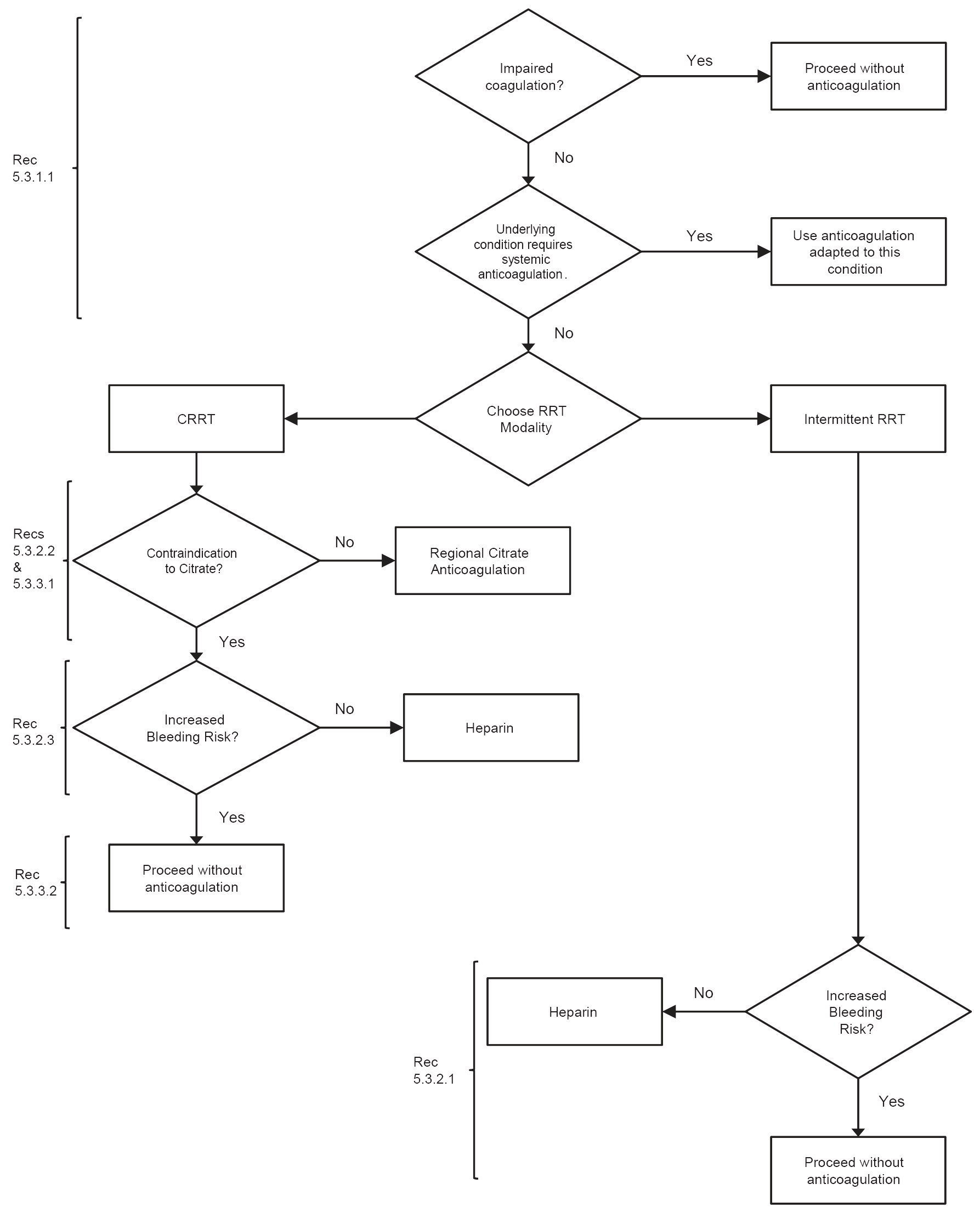
Figure 17 | Flow-chart summary of recommendations. Heparin includes low-molecular-weight or unfractionated heparin. CRRT, continuous renal replacement therapy; RRT, renal replacement therapy.
this to IHD for AKI, although studies in this setting are lacking. In patients with AKI, the dose of heparin for IHD and the target aPTT should be individualized according to the presence or absence of coagulation abnormalities and/or risk of bleeding.588,589 Monitoring should also include platelet count, allowing timely detection of HIT.581 Since low-molecular-weight heparins rely on the kidney as primary route of elimination, patients with kidney injury are at risk of accumulation and bleeding complications, depending on the degree of kidney injury, and the dose and type of low-molecular-weight heparin.590 The American College of Chest Physicians (ACCP) guidelines for antithrombotic and thrombolytic therapy therefore suggest using unfractionated instead of low-molecular-weight heparin in patients with severe renal insufficiency (CrCl < 30 ml/min) who require therapeutic anticoagulation, or to reduce the dose of lowmolecular-weight heparin by 50%.580 The doses of lowmolecular-weight heparin that are required for IHD are
Table 19 | Overview of the advantages and disadvantages of different anticoagulants in AKI patients
| Anticoagulant | Advantage | Disadvantage | References |
|---|---|---|---|
| Heparin (unfractionated) | Wide availability | Narrow therapeutic index – risk of bleeding | 580,581 |
| Large experience | Unpredictable kinetics – monitoring required | ||
| Short half-life | HIT | ||
| Antagonist available | Heparin resistance | ||
| Monitoring with routine tests | |||
| (aPTT or ACT) | |||
| Low costs | |||
| Low-molecular-weight heparin | More predictable kinetics | Risk of accumulation in kidney failure | 580, 582–584 |
| – Weight-based dosing possible | |||
| More reliable anticoagulant response | Monitoring requires nonroutine test (anti–Factor Xa) | ||
| – No monitoring required | |||
| Single predialysis dose may be sufficient in IHD |
Different drugs not interchangeable | ||
| Reduced risk of HIT | Incomplete reversal by protamine | ||
| In most countries more expensive than unfractionated heparin | |||
| Citrate | Strict regional anticoagulation – reduced bleeding risk |
Risk of accidental overdose with potentially fatal consequences | 585 |
| Insufficient citrate metabolism in patients with reduced liver function and shock states resulting in accumulation with metabolic acidosis and hypocalcemia | |||
| Other metabolic complication (acidosis, alkalosis, hypernatremia, hypocalcemia, hypercalcemia) | |||
| Increased complexity | |||
| Requires strict protocol |
aPTT, activated partial thromboplastin time; ACT, activated clotting time; HIT, heparin-induced thrombocytopenia; IHD, intermittent hemodialysis
lower than those required for therapeutic anticoagulation. The doses of low-molecular-weight heparin, as provided by the manufacturers, should be adapted to the bleeding risk of the individual patient. Dose reduction may also be required in patients receiving daily dialysis, which increases the risk of accumulation. Since many patients with AKI require prophylaxis for deep-vein thrombosis, scheduling this prophylactic (or a slightly higher) dose at the beginning of the dialysis session may serve the two purposes. Periodic measurement of anti–Factor Xa levels may be useful with prolonged use.
Alternative anticoagulants for IHD include protease inhibitors such as nafamostate and platelet inhibitors such as prostacyclin or its analogues. Randomized trials comparing these anticoagulants/antiaggregants with heparin in the setting of IHD for AKI are not available, and their use in clinical practice is limited. Nafamostat is a protease inhibitor that is mainly used in Japan and not available in the USA or Europe. Small observational trials in chronic dialysis patients with increased bleeding risk suggest a reduced bleeding incidence.591-593 Concerns with nafamostat include the absence of an antidote, and side-effects such as anaphylaxis, hyperkalemia, and bone marrow suppression.594-596 Crossover comparisons of prostacyclin with low-molecular-weight heparin in chronic dialysis patients show reduced efficiency.597 A small trial showed reduced bleeding complications compared to low-dose heparin; however, at the expense of slightly more premature terminations.598 Additional drawbacks are systemic hypotension and the high costs. Therefore, the routine use of alternative anticoagulants can not be recommended in patients with AKI.
The anticoagulant effect of sodium citrate relies on forming a complex with ionized calcium, thus removing an essential component of the coagulation cascade. Part of the citrate is removed in the extracorporeal circuit. Citrate reaching the systemic circulation is rapidly metabolized in the liver, muscle, and kidney, liberating the calcium and producing bicarbonate. The buffering effect of sodium citrate is proportional to the sodium ions it contains: a mole of trisodium citrate produces the same buffering effect as 3 moles of sodium bicarbonate; whereas preparations of citrate, including hydrogen citrate, have proportionally less buffering effect. Extracorporeal losses of calcium have to be compensated by an exogenous infusion. Additional complications of citrate are summarized in Table 19. Regional citrate anticoagulation requires a strict protocol, adapted to the local treatment modality and flow settings. The protocol should include instructions for the infusion of citrate and calcium, for the composition of the dialysate/replacement fluid, and for intensive metabolic monitoring, including acid-base status, sodium, and total and ionized calcium levels.
Five randomized trials have compared citrate to heparins during CRRT (Suppl Tables 31 and 32). For ethical reasons, these trials were performed in patients without increased bleeding risk. The first trial by Monchi et al. used a crossover design to compare anticoagulation with unfractionated heparin or citrate in 20 patients treated with postdilution CVVH. Patients with high bleeding risk, liver cirrhosis, and sensitivity to heparin were excluded. Forty-nine filters were evaluated. Citrate was titrated to achieve a postfilter ionized calcium level below 1.20 mg/dl (0.3 mmol/l). The dosing regimen of heparin consisted of a bolus of 2000 to 5000 U, followed by a continuous infusion of 500–2000 U/h, aiming at an aPTTof 60–80 seconds. Despite this rather high heparin dose, the citrate group had a longer filter lifetime and less spontaneous filter failure. Fewer patients in the citrate group required transfusion, and the number of transfused units was also lower. One patient in the heparin group experienced bleeding and one patient in the citrate group had metabolic alkalosis.599
The second trial randomized 30 patients with AKI undergoing predilution continuous venovenous hemodiafiltration (CVVHDF) to anticoagulation with citrate or unfractionated heparin. Patients with contra-indications to one of the two anticoagulants (mainly high bleeding risk/severe coagulopathy or metabolic problems that might be aggravated by citrate) or who required systemic anticoagulation for medical reasons were excluded. Heparin was titrated to achieve an aPTT of 45–65 seconds. Citrate was titrated to a postfilter ionized calcium between 1.0–1.40 mg/dl (0.25–0.35 mmol/l). Two patients in each group crossed over to the other anticoagulant and these filters were not included in the analysis. The trial was stopped early after 79 filters because of an advantage using citrate, which resulted in a significantly improved filter survival (124.5 hours vs. 38.3 hours; P < 0.001). In addition, significantly less citrateanticoagulated filters were terminated for clotting (16.7% vs. 53.5%). The incidence of bleeding also tended to be lower with citrate (RR 0.17; CI 0.03–1.04; P = 0.06), but transfusion requirement was not significantly different. Three patients in the citrate group had metabolic alkalosis and two had hypocalcemia.600
The third trial randomized 48 patients with AKI, treated with CVVH, to citrate or unfractionated heparin. Patients requiring systemic anticoagulation for medical reasons and patients with high bleeding risk, severe coagulopathy, circulatory failure, liver failure, or hypocalcemia were excluded (n = 12). A total of 142 circuits was analyzed. Heparin was administered as a bolus of 3000–5000 U followed by a continuous infusion of 1500 U/h adjusted to achieve an aPTTof 50–70 seconds. Citrate (500 mmol/l) was titrated to a postfilter ionized calcium between 1.0–1.20 mg/dl (0.25–0.30mmol/l). Neither circuit survival nor the reasons for disconnecting the CVVH circuit differed significantly between the two groups. However, the number of major bleedings and the need for transfusion was significantly greater in the heparin group. Two cases of metabolic alkalosis were noted in the heparin group and two episodes of hypocalcemia in the citrate group.601 Findings from two studies published after the cut-off date for our literature review are consistent with recommendation 5.3.2.2.601a,601b
A small randomized crossover study compared citrate anticoagulation to regional heparinization in 10 CVVH patients. Both treatment arms had a relatively short filter life (13 hours for regional heparinization and 17 hours for citrate) that did not differ significantly. No bleeding occurred in either group.602
In the largest and most recent randomized trial, 200 patients treated with postdilution CVVH were randomized to citrate or the low-molecular-weight heparin, nadroparin. Again, patients with bleeding risk or liver cirrhosis were excluded. Nadroparin was started with a bolus of 2850 U followed by 380 U/h without further monitoring. Citrate (500mmol/l) was administered at a dose of 3mmol per liter blood flow, without monitoring of postfilter ionized calcium. The primary outcomes were safety, defined as the absence of adverse events necessitating discontinuation of the study anticoagulant, and efficacy, defined as circuit survival. Safety was significantly better in the citrate group with only two patients requiring a change in anticoagulation regimen vs. 20 patients in the nadroparin group (P > 0.001). Adverse events were citrate accumulation (n = 1) and early clotting due to protocol violation (n = 1) in the citrate group, and bleeding (n = 16) or severe thrombocytopenia (n = 4) in the nadroparin group. Circuit survival did not significantly differ. A computerdriven combination of buffered and nonbuffered replacement fluids was used in the citrate group, explaining why metabolic alkalosis occurred more frequently in the nadroparin group. Rather surprisingly, the authors also found an improved renal recovery and an improved hospital survival in the citrate group. This could not be attributed to differences in severity of illness, nor in bleeding or transfusion requirement, and requires further investigation.603
Metabolic complications were infrequent in these randomized trials. In observational trials, the most frequent metabolic complication is metabolic alkalosis, occurring in up to 50% of the patients.604–606 In recently published surveys or large clinical trials, the use of regional citrate anticoagulation is still limited to 0–20% of the patients/treatments.562,563,607
A major contra-indication for the use of citrate anticoagulation is severely impaired liver function or shock with muscle hypoperfusion, both representing a risk of citrate accumulation. Markedly reduced citrate clearances and lower ionized calcium levels have been found in patients with acute liver failure or with severe liver cirrhosis.608–610 These patients were excluded in all the randomized trials. In patients at risk, intensified monitoring is recommendable. The ratio of total to ionized calcium appears to be the best parameter to detect citrate accumulation611,612 with an optimal cutoff at 2.1.613 Another important drawback of citrate anticoagulation, that might influence the decision to implement it in routine clinical practice, is the increased complexity of the procedure, with risk of metabolic complications and the need for a strict protocol adapted to the local RRT modality. We, therefore, only recommend the use of citrate for anticoagulation during CRRT in patients that do not have shock or severe liver failure, and in centers that have an established protocol for citrate anticoagulation.
Unfractionated heparin still remains the most widely used anticoagulant during CRRT,562,563,607 mostly administered as a prefilter infusion, with large variability in the administered doses. When choosing a dose of heparin, the clinician should realize that the relationship among heparin dose, aPTT, filter survival, and bleeding complications is not straightforward,574,614–619 but it is common practice to measure aPTT for safety reasons and to adapt the target to the bleeding risk of the patient.
Only two small prospective RCTs have compared unfractionated to low-molecular-weight heparin for anticoagulation during CRRT in patients with AKI and, thus, no firm recommendations can be made. The first trial randomized 47 patients with AKI or systemic inflammatory response syndrome undergoing CVVHDF to heparin, starting with a bolus of 2000–5000 U followed by an infusion of 10 U/kg/h titrated to an aPTT of 70–80 seconds, or to dalteparin administered as bolus of 20 U/kg followed by an infusion of 10 U/kg/h. The mean aPTT in the heparin groupwas 79 seconds. The mean anti–Factor Xa level, determined in six patients in the dalteparin group, was 0.49 U/ml. Only 37 of the 82 tested filters were stopped for coagulation. There was no difference in filter survival (with electively discontinued filters being censored). The mean time to filter failure was 46.8 hours in the dalteparin group and 51.7 hours in the heparin group (NS). Three patients in each group had bleeding, with no difference in transfusion requirement between the two groups. Daily costs, including the coagulation assays, were 10% higher with dalteparin.620
The second trial used a crossover design in 40 patients with normal coagulation parameters undergoing predilution CVVH. Patients treated with unfractionated heparin received a bolus of 30 U/kg followed by a continuous infusion of 7 U/kg/h titrated to achieve an aPTT of 40–45 seconds. Enoxaparin was given as an initial bolus of 0.15 mg/kg followed by a continuous infusion of 0.05 mg/kg/h, adjusted to an anti–Factor Xa level of 0.25–0.30 U/ml. In the 37 patients that completed both treatment arms, mean filter life was 21.7 hours with heparin and 30.6 hours with enoxaparin (P = 0.017). A similar difference was found in the perprotocol analysis. The incidence of bleeding was low and not different between the two anticoagulants. Filter life did not correlate with aPTT or anti–Factor Xa level. Costs were similar in the two groups.616 Interestingly, these clinical studies did not find a correlation between anti–Factor Xa levels and filter life, questioning the value of anti–Factor Xa monitoring with regard to efficacy.616,621 However, if used for more than a few days, monitoring might be useful to detect accumulation.
Alternative anticoagulants for use during CRRT include the protease inhibitor nafamostate and the platelet inhibitors, prostacyclin and analogues. Both have a short half-life and a low MW, with the theoretical advantage of extracorporeal elimination and reduced systemic anticoagulation. Nafamostat is not available in the USA and Europe; there is no antidote and several side-effects (agranulocytosis, hyperkalemia, anaphylactoid reactions) have been described.594-596 A few small trials showed improved filter survival during CRRT when adding prostaglandins to heparin compared to heparin alone.622-644 However, prostaglandins appear to have a limited efficacy when used alone, induce systemic hypotension,625,626 and are expensive. Their use during CRRT can therefore not be recommended.